Savoya Simone Joyner - Best Explanation of Complicated Subject
In the US, 42% of adults are living with obesity, and among these individuals, two-thirds are women and one-third are men. One question that has been understudied and difficult to answer is “Why are women disproportionately affected by obesity?” The answer isn’t about willpower. It is about women’s health and hormones, which are often ignored by the scientific community.
For decades, obesity research has treated all bodies as if they function the same. But the hormone estradiol, the primary form of estrogen, reveals a different narrative. When estradiol levels drop, especially after menopause, the body’s natural defense against obesity weakens. This is mediated by estradiol’s ability to help the brain protect against diet-induced weight gain.
What we call “obesity” isn’t just calories in, calories out. It’s the result of a constant conversation between the gut, brain, immune system, and hormones, and this changes across a woman’s lifespan.
When a diet rich in fat and sugar is consumed, immune cells in the brain, called microglia, can become overactive and produce a state of neuroinflammation. Microglia are the brain’s clean-up crew, their cell processes can pick up debris and aid in overall brain health. When this process is disrupted, the brain’s hunger and fullness signals become dysregulated. That’s where estradiol steps in: it calms inflammation and keeps the brain’s communication about food in balance. This begins to give us a better understanding of why post-menopausal women often struggle to regulate appetite even when they eat sensibly.
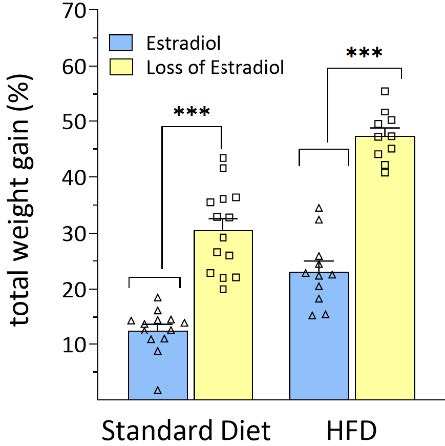
In my dissertation research using rodent models, we see this play out clearly. When female rats lose estradiol, mimicking menopause, they have a greater percentage of weight gain on both a standard diet and high-fat, high sugar diet in comparison to females with normal levels of estradiol (Figure 1). This difference is striking.
Research into sex differences opens the door to therapies that could mimic estradiol’s protective effects, without the risks of traditional hormone replacement. Yet only 5% of neuroscience studies published in 2019 directly investigated the influence of sex on diet induced weight gain and diseases. That’s not just a gap in knowledge but rather inequity in medical treatment.
For too long, women’s hormones have been discounted as “confounding variables,” something to control for rather than study. Hormones are not noise, they’re data. They help shape how the brain and body respond to diet, stress, and disease. If we continue this path of excluding female biology in research, we will build a science industry and research community that will continue to be inequitable for everyone.
The path forward is clear:
1. Require sex-inclusive research designs in all funded studies and expand neuroimmune research on how sex hormones protect the brain and body.
2. Train the next generation of scientists to include, rather than discount, female physiology in their study designs.
In the long term, understanding how estradiol influences the brain could help us fight obesity, Alzheimer’s disease, depression, and other conditions where inflammation and hormonal changes collide.
Estradiol’s story isn’t only about one hormone: it’s about rewriting how science values women’s health. If we truly care to tackle the obesity crisis and move toward personalized medicine, we need to study women’s brains and bodies with the urgency and respect they deserve.
